2023 Volume 48 Issue 4 Pages 211-219
2023 Volume 48 Issue 4 Pages 211-219
Products used in daily life contain multiple chemicals capable of inducing endocrine disruption in animals, including humans. One such typical substance is bisphenol A (BPA). BPA has been widely used in epoxy resins and polycarbonate plastics and can exert several adverse effects. Furthermore, given their structural similarity to BPA, phenolic analogs of BPA, i.e., synthetic phenolic antioxidants (SPAs), are considered to exhibit similar toxicity; however, the effects of early SPA exposure on the adult central nervous system remain poorly clarified. In the present study, we aimed to evaluate and compare the neurobehavioral effects of early life exposure to BPA and two selected SPAs, 4,4'-butylidenebis (6-tert-butyl-m-cresol) (BB) and 2,2'-methylenebis (6-tert-butyl-p-cresol) (MB). We exposed mice to low levels of these chemicals through drinking water during prenatal and postnatal periods. Subsequently, we examined the adverse effects of these chemicals on the central nervous system using a mouse behavioral test battery, comprising the open field test, light/dark transition test, elevated plus-maze test, contextual/cued fear conditioning test, and prepulse inhibition test, at 12-13 weeks old. Based on the behavioral analysis, SPAs, like BPA, may cause affective disorders even at low doses, although qualitative differences were noted in anxiety-related behaviors. In conclusion, our findings could be valuable for clarifying the potential adverse developmental risks of SPA exposure in early life.
It is well-known that daily-use products contain multiple chemicals, including flame retardants and photoinitiators, that are released into the environment through volatilization and abrasion (Yao et al., 2021; Li et al., 2020b). These chemicals may cause endocrine disruption in animals, including humans. One such typical substance is bisphenol A (BPA; CAS 85-05-7), a high-production volume chemical widely employed in epoxy resins and polycarbonate plastics. In humans, BPA exposure has been linked to several adverse effects, including reproductive and developmental disturbances, obesity, immune dysfunction, and psychiatric disorders (Feng et al., 2018; Pinney et al., 2017; Zhang et al., 2019; Berger et al., 2019; Metwally et al., 2018). Given that embryonic and infant periods are crucial for the development of the nervous system and brain morphology, adverse effects on neural development following early-life BPA exposure are well-established. Importantly, animal studies have shown that perinatal exposure to BPA inhibits neurogenesis in the hippocampal dentate gyrus (Komada et al., 2020), increases depression-like behavior with neurotransmitter and neuroactive steroid dysfunction (Xin et al., 2018), and induces anxiety-like behavior with a decrease in excitatory and inhibitory synaptic density (Kumar and Thakur, 2017). One major concern is that the effects of BPA are often non-monotonic and dose-independent, and neurobehavioral toxicity may occur even at low doses of exposure (Kundakovic et al., 2013; Gonçalves et al., 2010).
Although BPA usage persists in certain products, it has been regulated in several countries (e.g., the EU, United States [U.S.], China, and Canada), and there has been a growing shift to alternative substances. For example, bisphenol S (BPS) and bisphenol F (BPF) have replaced BPA, and such products are labeled “BPA-free”. However, these alternatives are unsafe for organisms, given that their biological effects may be similar to those of BPA (Harnett et al., 2021).
Owing to their structural similarity to BPA, phenolic analogs of BPA could potentially exert similar toxicity (Kotula-Balak et al., 2013; Yang et al., 2018b). Numerous phenol-based structural analogs of BPA exist and are incorporated in a wide range of products, including synthetic phenolic antioxidants (SPAs) (e.g., food, plastic, and rubber products). The incorporation of SPAs is a common strategy for retarding oxidation reactions in polymeric materials.
Like other additives, SPAs have a high potential for leaching into the environment. Several SPAs have been detected in environmental matrixes (e.g., river water, wastewater, dust, sludge, and sediment) (Wang et al., 2021). Thus, it can be assumed that SPA exposure occurs similarly to BPA exposure; however, the effects of SPAs on mammalian brain function remain poorly investigated. To the best of our knowledge, few studies have focused on behavior after maturation as an effect of early-life SPA exposure.
Herein, we selected two types of SPAs: 4,4'-butylidenebis (6-tert-butyl-m-cresol) (BB; CAS 85-60-9) and 2,2'-methylenebis (6-tert-butyl-p-cresol) (MB; CAS 119-47-1). BB is used in natural and synthetic rubber and latex. According to the U.S. Environmental Protection Agency (EPA), the BB production volume in the U.S. is estimated at 100,000–500,000 lb (National Center for Biotechnology Information, 2022a). BB might influence the functional development of the rat brain, as it was found to increase aromatase activity, which is associated with sexual differentiation and sexual behavior (Satoh et al., 2010). MB is used in acrylonitrile butadiene styrene, polypropylene, polyacetal, rubber, latex, and adhesives. The U.S. production of MB is estimated at 1,000,000–10,000,000 lb (National Center for Biotechnology Information, 2022b). MB induces E2 elevation in H295R cells and can potentially inhibit normal steroidogenesis (Yang et al., 2018a). These substances are widely employed by professionals and consumers and are included in the Community Rolling Action Plan (CoRAP) by the European Chemicals Agency (ECHA, 2022a, b), raising safety concerns regarding exposure-induced effects.
In the present study, we assessed and compared the neurobehavioral effects of early-life exposure to BPA and two selected SPAs (BB and MB) to elucidate the potential developmental implications of perinatal exposure to them, using 10 times the tolerable daily intake (TDI) for BPA.
Figure 1 outlines the experimental protocol. Pregnant female C57BL/6N mice at embryonic day (E)-11.5 were purchased from Japan SLC (Shizuoka, Japan). BPA (Kanto Chemical Co., Inc., Tokyo, Japan), BB (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan), and MB (Tokyo Chemical Industry Co., Ltd.) were dissolved in ethanol and subsequently in drinking water to achieve an ethanol concentration of 0.0001%. The mice were divided into four groups: vehicle control (Vehicle), BPA, BB, and MB. The Vehicle group was treated with 0.0001% ethanol in water. To the best of our knowledge, the TDI values for BB and MB have not been defined, whereas the U.S. EPA has established a TDI of 50 μg/kg/day for BPA. In a previous study, perinatal exposure to BPA at 500 μg/kg/day (10 times higher than the TDI) was found to cause neurobehavioral abnormalities (Tian et al., 2010; Ogi et al., 2013); therefore, three exposure groups of animals were treated with each chemical substance in drinking water at 5 ppm to achieve the targeted dose levels of 500 μg/kg/day. Dams were exposed to BPA, BB, and MB from the gestation period (E11.5) to maternal ablactation when the pups were 4 weeks old. After maternal ablation, four male pups were selected from each dam and housed in separate cages. The pups used for the behavioral test battery were selected from two or three litters in each group. Mice were housed in a room at constant temperature (24 ± 1°C) and humidity (60 ± 10%) with a 12 hr light/dark cycle. All animals had free access to food and water. All animal care and experimental procedures were conducted in accordance with the Regulations for Animal Experiments and Related Activities at the National Institute of Health Sciences. This study was approved by the Animal Ethics Committee of the National Institute of Health Sciences (Permission No. 65-1).
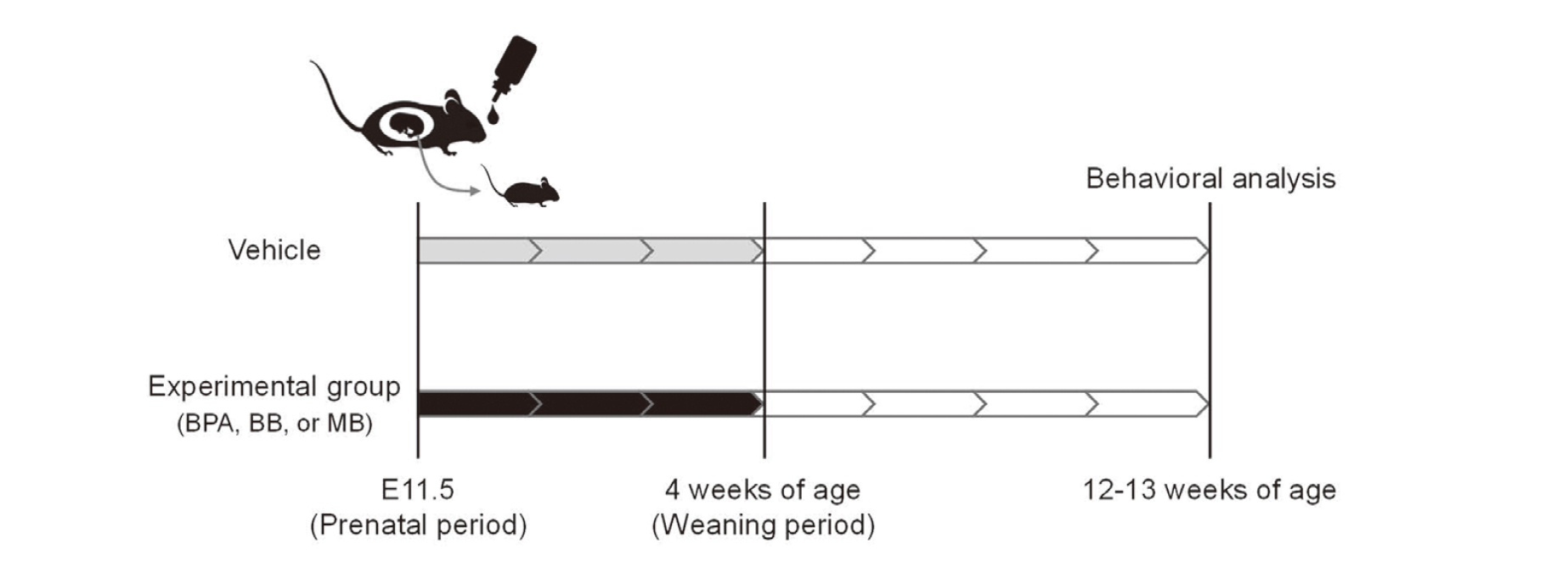
Schematic diagram illustrating the chemical exposure protocol. Gray: administration of vehicle by drinking water, Black: administration of chemical by drinking water, White: no treatment. Vehicle: vehicle control group, BPA: bisphenol A exposure group, BB: 4,4'-butylidenebis (6-t-butyl-3-methylphenol) exposure group, MB: 2,2'-methylenebis (6-tert-butyl-p-cresol) exposure group.
The behavioral test battery was conducted using 12–13 weeks old mice (Vehicle; n = 8, BPA; n = 8, BB; n = 8, MB; n = 9). We performed a behavioral test battery comprising the open field (OF) test, light/dark transition (LD) test, elevated plus-maze (EP) test, contextual/cued fear conditioning (FZ) test, and prepulse inhibition (PPI) test. Tests were performed as previously described (Tanemura et al., 2009; Saito et al., 2019), with some modifications. The experimental apparatus and image analysis software (ImageJ OF4, ImageJ LD2, ImageJ EP2, ImageJ FZ2, and SR-9040) were obtained from O’Hara and Co. Ltd (Tokyo, Japan). ImageJ OF4, LD2, EP2, and FZ2 were developed using ImageJ. The level of background noise during several behavioral tests was approximately 50 dB.
OF test: Locomotor activity was measured for 10 min using a white plastic OF apparatus (50 × 50 × 40 [H] cm). The LED light system was positioned approximately 60 cm above the center of the field (50 lx at the center of the field). Behavior was measured using a charge-coupled device (CCD) camera positioned 50 cm above the center of the OF apparatus.
LD test: For the LD test, the apparatus consisted of a cage (21 × 42 × 25 [H] cm) divided into two chambers by a partition with an opening. One chamber was composed of white plastic and was brightly illuminated (250 lx, light area), whereas the other chamber, made of black plastic, was dark (5 lx, dark area). Behavior was measured using a CCD camera positioned above each chamber. The mouse was placed in the dark and allowed to move freely between the two chambers using the opening for 5 min.
EP test: The plus-shaped apparatus consists of four arms (25 × 5 cm) connected to a central square area (5 × 5 cm). Two opposing arms were enclosed with 20 cm high transparent walls, whereas the other two were left open. The maze floor was made of white plastic and was elevated 50 cm above the room floor (10 lx at the center of the apparatus). Behavior was measured using a CCD camera positioned above each chamber. A mouse was placed in the central square area of the maze, facing one of the arms, and behavior was recorded for 5 min.
FZ test: The apparatus consisted of a conditioning chamber (test chamber: 17 × 10 × 10 [H] cm) made of clear plastic with a ceiling. The chamber floor had stainless steel rods (2 mm diameter) spaced 5 mm apart to impart electric foot shock to mice. The LED light system was positioned 50 cm above the chamber floor (100 lx at the center of the floor). Behavior was measured using a CCD camera positioned 20 cm above the ceiling of the chamber. During the conditioning trial (day one), mice were placed individually in the conditioning chamber; after 90 sec, they received three tone shock pairings (30 sec of tone at 65 dB, 10 kHz directly followed by 3 sec of 0.1 mA electric shock), each separated by 120 sec. Mice were then returned to their home cage. On the next day (day two), mice were returned to the conditioning chamber for 6 min without tone or shock to examine contextual fear. As a cued fear test on the third day (day three), mice were placed in a novel chamber (with a different design, lacking stainless steel rods, 50 lx at the center of the floor). After 3 min, a conditioning tone (with no shock) was presented for 3 min. ImageJ FZ2 measured the freezing response of the mice as a consecutive 2 sec period of immobility. The freezing rate (%) was calculated as follows: (freezing/session time) × 100.
PPI test: The apparatus consisted of a light source, sound system, and a startle measurement load cell, which was placed in a soundproof box. The white background noise level in the soundproof box was set to 70 dB. The test schedule consisted of three blocks, and the total trial duration was 30 min. Each block was broken down as follows: 80–, 85–, 90–, 95–, 100–, 105, and 110 dB pulse × 3 (acclimation block) and 120 dB pulse × 10 (acoustic startle response block). The prepulse combinations were 80–120, 85–120, 90–120, 95–120, 100–120, and 105–120 dB, with a delay of 100 ms × 6 (PPI measurement block). These combinations were presented in a pseudorandom order, such that each trial type was presented once within a block. The inhibition ratio (%) of the startle response was calculated as follows: (1-prepulse [80, 85, 90, 95, 100, or 105 dB] startle response value/acoustic startle response value) × 100.
Statistical analysisData analyses were performed using Dunnett’s multiple comparisons test with KyPlot version 6.0 (KyensLab Inc., Tokyo, Japan). P-values < 0.05 were considered statistically significant (*P < 0.05, **P < 0.01).
No clinical signs of BPA, BB, or MB were observed during or after treatment in either dams or offspring. No pup deaths were observed until weaning. Moreover, mice appeared to grow normally. In addition, we noted no significant differences in body weight between experimental groups and their respective vehicle groups, both before and after the behavioral test battery (Table 1).
| Treatment groups |
Body weight (g) | |||||
|---|---|---|---|---|---|---|
| Before behavioral test battery (12 weeks) |
After behavioral test battery (13 weeks) |
|||||
| Vehicle | 28.1 | ± | 1.8 | 28.6 | ± | 1.8 |
| BPA | 28.0 | ± | 2.3 | 28.4 | ± | 2.2 |
| BB | 26.9 | ± | 1.9 | 27.4 | ± | 1.6 |
| MB | 29.8 | ± | 2.3 | 30.1 | ± | 2.4 |
Data are expressed as mean ± standard deviation (S.D.) and were tested statistically using Dunnett’s multiple comparisons test. Vehicle: vehicle control group (n = 8), BPA: bisphenol A exposure group (n = 8), BB: 4,4'-butylidenebis (6-t-butyl-3-methylphenol) exposure group (n = 8), MB: 2,2'-methylenebis (6-tert-butyl-p-cresol) exposure group (n = 9).
The total distance was significantly decreased in the MB group when compared with the Vehicle group (Vehicle: 3389.6 ± 194.5, BPA: 3246.8 ± 134.7, BB: 2791.8 ± 242.8, MB: 2432.5 ± 99.6; Fig. 2A). In addition, the total time spent in the center did not differ significantly in all exposure groups (Vehicle: 122.8 ± 9.0, BPA: 131.4 ± 13.5, BB: 129.3 ± 19.0, MB: 105.3 ± 16.4; Fig. 2B). The number of movement episodes was significantly lower in the BB-exposed group (Vehicle: 188.5 ± 3.4, BPA: 181.8 ± 4.9, BB: 165.3 ± 8.0, MB: 185.0 ± 3.8; Fig. 2C).

Results of the open field (OF) test. Scores of the OF test (total test time, 600 sec) are displayed. (A) Distance traveled (cm) during the test period. (B) Time spent in the center area (sec). (C) Number of movements during the test period. Data are expressed as the mean ± standard error (S.E). Data were tested statistically using Dunnett’s multiple comparisons test. *P < 0.05 and **P < 0.01, compared with the Vehicle group. Vehicle: vehicle control group (n = 8), BPA: bisphenol A exposure group (n = 8), BB: 4,4'-butylidenebis (6-t-butyl-3-methylphenol) exposure group (n = 8), MB: 2,2'-methylenebis (6-tert-butyl-p-cresol) exposure group (n = 9).
In all exposure groups, no significant differences were observed in the time spent in the light chamber and latency to enter the light chamber when compared with the Vehicle group (Vehicle: 101.5 ± 9.9, BPA: 60.3 ± 12.7, BB: 85.1 ± 16.9, MB: 77.4 ± 11.1; Fig. 3A, Vehicle: 56.0 ± 15.6, BPA: 75.4 ± 24.7, BB: 70.1 ± 21.6, MB: 48.6 ± 6.4; Fig. 3C). The number of transitions was significantly altered in the BPA-exposed group (Vehicle: 13.4 ± 1.8, BPA: 5.8 ± 1.4, BB: 11.6 ± 2.2, MB: 8.0 ± 1.1; Fig. 3B).

Results of the light/dark transition (LD) test. Scores of the LD test (total test time, 300 sec) are displayed. (A) Time spent in the light chamber area (sec). (B) The number of transitions between the dark and light chambers. (C) Latency to enter the light chamber for the first time (sec). Data are expressed as the mean ± standard error (S.E). Data were tested statistically using Dunnett’s multiple comparisons test. *P < 0.05, compared with the Vehicle group. Vehicle: vehicle control group (n = 8), BPA: bisphenol A exposure group (n = 8), BB: 4,4'-butylidenebis (6-t-butyl-3-methylphenol) exposure group (n = 8), MB: 2,2'-methylenebis (6-tert-butyl-p-cresol) exposure group (n = 9).
In the EP test, we noted no significant changes in any exposure groups when compared with the Vehicle group. (Vehicle: 759.9 ± 55.1, BPA: 859.1 ± 76.2, BB: 889.6 ± 43.9, MB: 656.1 ± 37.8; Fig. 4A, Vehicle: 39.6 ± 12.2, BPA: 36.1 ± 8.2, BB: 53.3 ± 12.7, MB: 29.8 ± 11.8; Fig. 4B, Vehicle: 14.1 ± 1.3, BPA: 17.4 ± 2.0, BB: 17.9 ± 1.6, MB: 13.6 ± 0.9; Fig. 4C).

Results of the elevated plus-maze (EP) test. Scores of the EP test (total test time, 600 sec) are displayed. (A) Distance traveled (cm) during the test period. (B) Time spent in the open area (sec). (C) Total entry number in arms. Data are expressed as the mean ± standard error (S.E). Data were tested statistically using Dunnett’s multiple comparisons test. *P < 0.05, compared with the Vehicle group. Vehicle: vehicle control group (n = 8), BPA: bisphenol A exposure group (n = 8), BB: 4,4'-butylidenebis (6-t-butyl-3-methylphenol) exposure group (n = 8), MB: 2,2'-methylenebis (6-tert-butyl-p-cresol) exposure group (n = 9).
Considering the FZ test, no significant changes were detected in any exposure groups when compared with the Vehicle group (Vehicle: 10.7 ± 1.5, BPA: 15.7 ± 2.2, BB: 7.5 ± 1.6, MB: 9.1 ± 2.6; Fig. 5A, Vehicle: 30.9 ± 6.4, BPA: 43.0 ± 8.6, BB: 29.2 ± 4.6, MB: 33.2 ± 4.9; Fig. 5B, Vehicle: 53.4 ± 5.7, BPA: 43.7 ± 5.1, BB: 43.0 ± 5.1, MB: 45.8 ± 4.9; Fig. 5C).
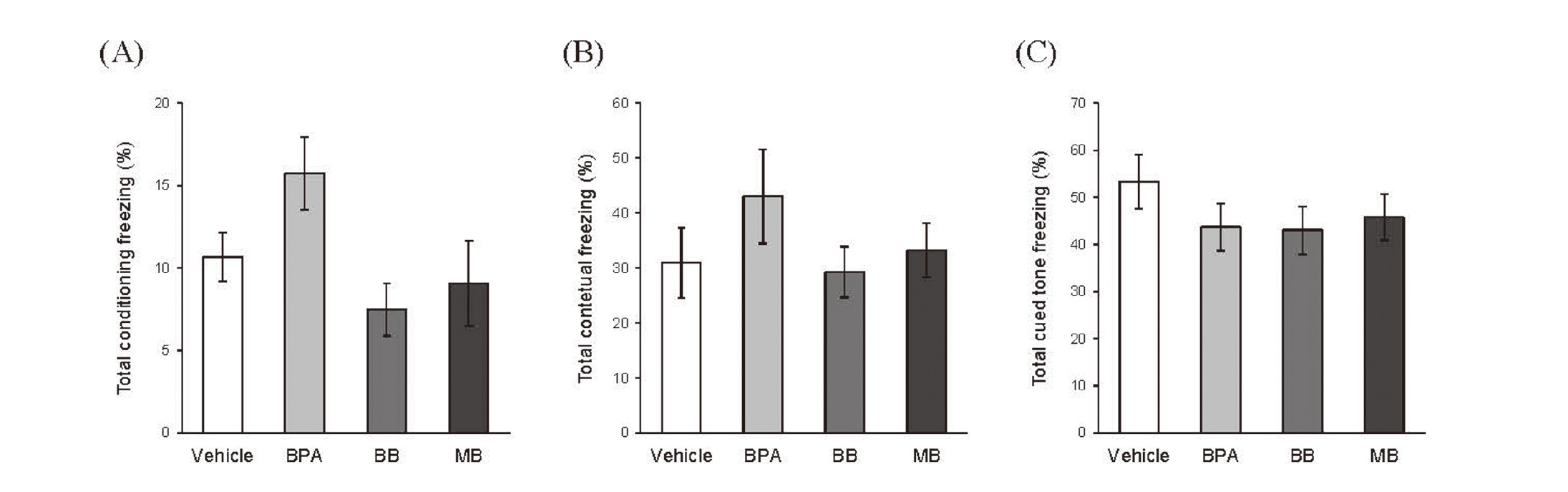
Results of the contextual/cued fear conditioning (FZ) test. The total test time was 360 sec. (A) FZ was conducted to analyze the effects of chemicals on learning and memory, i.e., place (context) and sound (cued tone). The average total freezing scores (%) of the Vehicle and all exposure groups in the conditioning test are shown. (B) The contextual test was conducted to analyze the effects of chemicals on place memory function. The average total freezing scores (%) of the Vehicle and all exposure groups in the contextual test are shown. (C) The cued test was used to analyze the effects of chemicals on cued memory function. The average freezing scores (%) of the Vehicle and all exposure groups after presenting the tone. Data are expressed as the mean ± standard error (S.E). Data were tested statistically using Dunnett’s multiple comparisons test. *P < 0.05, compared with the Vehicle group. Vehicle: vehicle control group (n = 8), BPA: bisphenol A exposure group (n = 8), BB: 4,4'-butylidenebis (6-t-butyl-3-methylphenol) exposure group (n = 8), MB: 2,2'-methylenebis (6-tert-butyl-p-cresol) exposure group (n = 9).
In the PPI test, we documented no significant changes in any exposure group when compared with the vehicle group (Vehicle: 48.9 ± 17.6, BPA: 72.9 ± 8.0, BB: 74.6 ± 10.3, MB: 45.0 ± 31.2; Fig. 6A, Vehicle: 88.4 ± 2.0, BPA: 91.5 ± 3.1, BB: 93.1 ± 1.6, MB: 87.82 ± 5.35; Fig. 6B).

Results of the prepulse inhibition (PPI) test. Representative scores are displayed. (A and B) The inhibition (%) of the startle response to a 120 dB sound with prepulse sounds of 95 and 100 dB compared with the response to a 120 dB sound without a prepulse sound. Data are expressed as the mean ± standard error (S.E). Data were tested statistically using Dunnett’s multiple comparisons test. *P < 0.05, compared with the Vehicle group. Vehicle: vehicle control group (n = 8), BPA: bisphenol A exposure group (n = 8), BB: 4,4'-butylidenebis (6-t-butyl-3-methylphenol) exposure group (n = 8), MB: 2,2'-methylenebis (6-tert-butyl-p-cresol) exposure group (n = 9).
It should be noted that perinatal chemical exposure, even at low concentrations that are generally considered safe for humans, may have adverse effects on neurodevelopment, sexual differentiation, emotional behavior, learning and memory, and social behavior (Xu et al., 2011; Fujimoto et al., 2006; Vandenberg et al., 2012). Additionally, even minor effects should be considered as potential toxicity in safety evaluations. Considering that SPAs are as widely used as BPA, understanding their safety during development in early-life exposure is necessary. Therefore, we aimed to evaluate and compare the neurobehavioral effects of early life exposure to BPA, along with two selected SPAs (BB and MB).
Perinatal BPA exposure can primarily induce anxiety-related behaviors in the offspring. Epidemiological studies have reported that BPA exposure during early life is associated with an increased risk of anxiety and depression in children, with the association being more pronounced in boys (Perera et al., 2016; Harley et al., 2013; Li et al., 2020a). The OF, LD, and EP tests are commonly employed to detect anxiety-related behaviors in mice and are based on the natural aversion of mice to a novel environment, brightly lit spaces, or elevation. In addition, it is recommended that multiple tests should be used to evaluate different types of anxiety-related behaviors, as these results are not always consistent (Takao and Miyakawa, 2006; van Gaalen and Steckler, 2000).
In the OF test, the total distance traveled was significantly reduced in the MB group, and the number of movement episodes was significantly decreased in the BB group. The results of the MB group indicate that spontaneous exploratory activity was reduced. Although the number of movement episodes in the BB group decreased, the total distance traveled remained unchanged, suggesting an increased in the distance traveled per episode. This indicates that restlessness may have led to this result. These behavioral changes of the MB and BB group could be attributed to the elevated anxiety associated with placement in a novel environment. However, anxiety due to fear of open spaces was not enhanced, given that the total time spent in the center did not significantly differ across exposure groups. In the LD test, the BPA group showed significantly fewer transitions. Additionally, the BPA group spent less time in the light chamber than did the other groups; however, the difference was not statistically significant. This finding may be attributed to mice experiencing high anxiety in the brightly lit space, therefore entering it less frequently. The results of the LD test are consistent with those of previous studies, reporting that maternal BPA exposure increases anxiety- and depression-like behavior in offspring (Xu et al., 2012; Tian et al., 2010). However, contrary to our expectations, behavioral abnormalities in the OF and EP tests observed in previous studies were not detected in the present study. These discrepancies could be induced by differences in chemical concentration, administration period, and subject age. The findings of this behavioral analysis revealed that, in comparison to BPA, BB and MB induce different types of anxiety-like behaviors. Accordingly, SPAs, like BPA, could potentially induce affective disorders even at low doses, although there are qualitative differences in anxiety-related behaviors.
The investigation of chemical toxicity in dose-response studies provided more detailed toxic effects. However, an increase in the number of subjects in behavioral analysis should be avoided because diurnal variation exists in rodent behaviors (Morales-Delgado et al., 2018; Meseguer Henarejos et al., 2020). We conducted behavioral studies with the smallest number of mice to minimize the confounding factors of diurnal variation in mouse behavior. Furthermore, the purpose of the present study was limited to identifying the behavioral risks associated with early-life low-dose chemical exposure. Although the results obtained using this experimental design have limitations, there is still room to collect additional dose-response findings using SPAs in the future.
Herein, we did not assess the histological and molecular effects of test substances on the brain; therefore, the underlying mechanism of neurobehavioral abnormalities induced by these chemicals remains unclear. As an endocrine disrupter, BPA is thought to impair the regulation of endogenous estrogen in the neural circuitry, resulting in anxiety- and depression-like behaviors (Xu et al., 2012). The limbic system, including the hippocampus and amygdala, is a potential target for the effects of estrogen on these behaviors (Walf and Frye, 2006). BPF and BPS, alternatives to BPA, have been shown to induce estrogenic effects (Park et al., 2020). Accordingly, BB and MB might possess estrogen-like effects and induced emotional disturbance via effects on the hippocampus and amygdala. In addition, BPA exposure-induced effects can differ based on sex (Xu et al., 2011; Xu et al., 2015; Thongkorn et al., 2021); however, further investigations are necessary to clarify whether sex differences impact the effects of BB and MB.
This study is first to provide evidence that adverse effects of early exposure to low doses of BPA and its phenol-based structural analogs may persist long after maturation, which manifested as rodent behaviors associated with anxiety and depression. In 2015, the European Food Safety Authority (EFSA) reduced the TDI of BPA from 50 to 4 μg/kg/day (EFSA CEF Panel, 2015). In 2021, the EFSA suggested that the TDI be dramatically reduced to 0.04 ng/kg/day (EFSA, 2021). Given these reassessed TDI values, it is critical to carefully establish chemical regulatory values and revise them based on additional research data.
In conclusion, despite certain limitations, our findings could help clarify the potential adverse developmental risks associated with early life exposure to SPAs. Although no dramatic behavioral changes were observed in this study, in addition to BPA, perinatal exposure to SPAs will be a new concern factor for brain development, particularly hippocampal and amygdala-related functions.
We thank Editage (www.editage.com) for the English language editing. This study was supported in part by Health Sciences Research Grants from the Ministry of Health, Labour, and Welfare, Japan (H30-KAGAKU-IPPAN-003), JSPS KAKENHI (Grant Number 19H01142), and JST SPRING [Grant Number JPMJSP2114].
Conflict of interestThe authors declare that there is no conflict of interest.